FOR DUCTILE, BRIGHT FINELY SMOOTHED ACID COPPER PLATING.
Copper used in phosphorus-acid copper anodes is oxygen-free and features a high degree of purity, which makes it ideal for use in electronics applications.
The addition of phosphorus to an oxygen-free copper base and the extremely low level of copper impurities ensure the use of the correct amount of phosphorus and thus predictable yielding. The base of this type of anodes is made of the same CuOF 2000 branded copper.
Phosphorus acid copper anodes are obtained from selected electrolytic copper cathodes melted in an induction furnace, in an oxygen-free atmosphere.
A controlled amount of a phosphorus master alloy is added to the molten copper, which is poured in a protective gas atmosphere. This exclusive process prevents the formation of harmful oxides or the inclusion of other impurities.
FOUR REASONS WHY THE USE OF PHOSPHORUS ACID COPPER ANODES ENSURES MAXIMUM PERFORMANCE.
1. LESS INSOLUBLE ELEMENTS MEANS SMOOTHER COPPER PLATING.
The combined effect of controlled phosphorus content and the absence of insoluble impurities prevents surface roughness. Since the amount of sludge produced is minimized, roughness caused by agitation of sludge particles is very unlikely to occur.
2. LESS SLUDGE MEANS LESS PLATING VAT MAINTENANCE.
The purity of copper anodes and controlled phosphorus content combine ideally to reduce to a minimum the amount of sludge involving the anode. Less sludge means less maintenance, and reduced dead times mean increased output.
3. EVEN DISSOLUTION TO REDUCE ANODE SCRAPPING.
Phosphorus copper anodes dissolve in a uniform manner, both in the solution and below surface. This means that the anode is used up completely. In other words, more copper is used for plating and less scrap is produced.
4. LESS POLISHING.
With phosphorus copper anodes, a thinner copper deposition can be obtained since the coating is smooth, even and dense. Uneven deposition, which often occurs with other types of anodes, requires a thicker and heavier copper plating and more expensive polishing to achieve the same smooth finish. With phosphorus copper anodes no polishing is required.
DATA SHEET – PHOSPHORUS ACID COPPER ANODES
BETTER BRIGHT COPPER PLATING AT LOWER PRICES.
Bright acid copper plating – in the broader context of a corrosion protection system – gives excellent results at reduced costs, thus ensuring the three requirements of brilliance, good ductility and exceptionally smooth surfaces. In order to maximize the results of a process based on brigh acid baths you need to use highly active phosphorous copper anodes.
Why phosphorus copper anodes?
In a galvanic bath, the copper anode is as important as the solution, the current or the cathode itself. The success of a copper plating process also depends of the proper functioning of the anode. Acid copper plating baths require copper anodes with a controlled content of phosphorus, usually
between 0.04% and 0.06%. This guarantees the achievement of four inter-acting objectives:
1. a shiny, smooth, dense and ductile finish
2. no copper enrichment in the bath
3. less formation of sludge in the plating vat
4. faster deposition
Since anode efficiency is higher than cathode efficiency, phosphorus-free copper anodes are dissolved in the acid bath faster than the amount absorbable by the cathode. This causes an accumulation of copper ions and consequence imbalance of the solution.
Moreover, phosphorus-free copper anodes produce a metal powder that settles at the bottom in the form of sludge. The problem of sludge accumulation gets worse when the anodes contain insoluble impurities and
copper oxides. The greater the anode impurity, the greater the problem of sludge.
Sludge can play a major role in creating roughness on the deposit. The injection of air in the baths can be a further vehicle that causes the sludge particles to migrate on the cathode together with the copper ions, thus creating a rough, unsmooth deposition. Moreover, sludge involves additional costs in terms of operations required to drain and recover the sludge and maintain the vat.
The Role of Phosphorus.
The presence of phosphorus reduces electrical conductivity, making it dissolve more slowly. A phosphorus anode will dissolved with lower anode efficiency than a phosphorus-free anode, bringing it closer to the optimal 100% cathode efficiency.
The presence of a proper amount of phosphorus inhibits the formation of copper ions, which may occur when phosphorus-free anodes are used in a sulphate bath.
Cupreous ions oxidize and form cupric ions and metallic copper, which produces copper powder that go to form the main part of the sludge. The sludge formed by phosphorus copper anodes is normally due to an insufficient amount of phosphorus, the presence of insoluble impurities in the copper, or the combination of both factors.
The solution to this problem is an even available amount of phosphorus.
Conventional phosphorus anodes are produced by adding phosphorus to electrolytic tough pitch (ETP) copper containing variable amounts of oxygen. Part of the phosphorus de-oxygenates the copper and will not bind with it.
Only a residual portion of the added phosphorus will be available. It is therefore difficult to determine in advance the amount of phosphorus available as this depends on the amount of oxygen contained in the refined copper, which normally varies between 0,025% and 0.05%.
Insoluble phosphorus pentoxides and other anode impurities contribute to the formation of roughness and sludge. Pores and low current density points can create copper particles.
Just as the content of phosphorus in the de-oxidized anodes with residual phosphorus varies from one batch to another, porosity and impurities varies accordingly.
DATA SHEET: PACKAGING FOR PHOSPHORUS COPPER ANODES
PHOS 2000 anodes are available in the shapes, sizes and packaging shown in the table below.
|
|||||||||||||
|
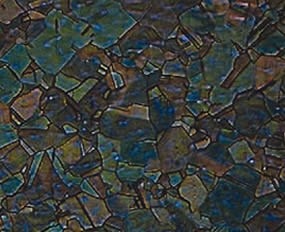
Micrografia di un anodo PHOS 2000 fuso

Micrografia di un anodo PHOS 2000 estruso